The R-fMRI Network (RFMRI.ORG):
A network for supporting resting-state fMRI related studies!
All human higher mental functions such as thinking, emotion and consciousness rely on the brain, an extremely complex system 1.
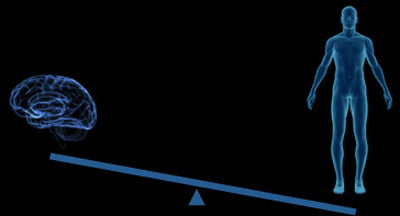
The brain accounts for only 2% of body weight, but receives 11% of cardiac output and consumes 20% of energy 2.
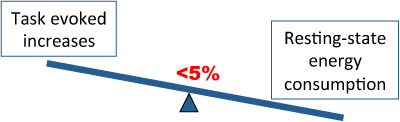
Compared with baseline energy consumption, task-evoked increases are less than 5%. The implication is that intrinsic activity, i.e., during resting-state, comprises important functions 3,4.
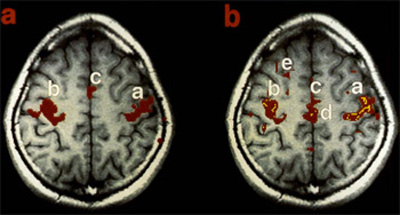
Although considered noise in traditional task-based fMRI studies, Biswal and colleagues first reported that spontaneous fluctuations of the resting-state fMRI (R-fMRI) signal were highly structured (See figure above from the 1995 paper) 5.

Since, R-fMRI has emerged as a mainstream imaging modality with myriad applications in basic, translational and clinical neuroscience 3,6-8. Beyond impressive demonstrations of accuracy, reliability and reproducibility for an increasing number of measures of intrinsic brain function 9-13, this approach has gained popularity due to its sensitivity to developmental, aging and pathological processes, e.g., 14-17, ease of data collection in otherwise challenging populations, and amenability to aggregation across studies and sites 18-21.
The R-fMRI Network (rfmri.org) has been designed as a framework to support R-fMRI studies. The R-fMRI Network comprises R-fMRI researchers (the nodes) who are connected by sharing (the edges) with each other. Through the network, we can efficiently share ideas, comments, resources, tools, experiences, data, and our increasing knowledge of the brain. Researchers (nodes) with basic neuroscience, methodological, or clinical backgrounds can connect with each other in the network. We hope the R-fMRI Network will help to enhance collaborations among researchers, especially to translate our knowledge of basic neuroscience and methodology to clinical applications (bench to bedside).
Hope you will enjoy The R-fMRI Network (RFMRI.ORG)!
1 Singer, W. Neuronal synchrony: a versatile code for the definition of relations? Neuron 24, 49-65, 111-125, doi:S0896-6273(00)80821-1 [pii] (1999).
2 Raichle, M. E. Two views of brain function. Trends in cognitive sciences 14, 180-190, doi:S1364-6613(10)00029-X [pii]
10.1016/j.tics.2010.01.008 (2010).
3 Fox, M. D. & Raichle, M. E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8, 700-711 (2007).
4 Zhang, D. & Raichle, M. E. Disease and the brain's dark energy. Nat Rev Neurol 6, 15-28, doi:nrneurol.2009.198 [pii]
10.1038/nrneurol.2009.198 (2010).
5 Biswal, B., Yetkin, F. Z., Haughton, V. M. & Hyde, J. S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34, 537-541 (1995).
6 Kelly, C., Biswal, B. B., Craddock, R. C., Castellanos, F. X. & Milham, M. P. Characterizing variation in the functional connectome: promise and pitfalls. Trends in cognitive sciences 16, 181-188, doi:10.1016/j.tics.2012.02.001 (2012).
7 Fornito, A. & Bullmore, E. T. Connectomic intermediate phenotypes for psychiatric disorders. Front Psychiatry 3, 32, doi:10.3389/fpsyt.2012.00032 (2012).
8 Van Dijk, K. R. et al. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. Journal of neurophysiology 103, 297-321, doi:10.1152/jn.00783.2009 (2010).
9 Shehzad, Z. et al. The resting brain: unconstrained yet reliable. Cereb Cortex 19, 2209-2229, doi:bhn256 [pii]
10.1093/cercor/bhn256 [doi] (2009).
10 Zuo, X. N. et al. The oscillating brain: Complex and reliable. Neuroimage 49, 1432-1445, doi:S1053-8119(09)01016-7 [pii]
10.1016/j.neuroimage.2009.09.037 (2010).
11 Damoiseaux, J. S. et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A 103, 13848-13853, doi:0601417103 [pii]
10.1073/pnas.0601417103 (2006).
12 Guo, C. C. et al. One-year test-retest reliability of intrinsic connectivity network fMRI in older adults. Neuroimage 61, 1471-1483, doi:10.1016/j.neuroimage.2012.03.027 (2012).
13 Thomason, M. E. et al. Resting-state fMRI can reliably map neural networks in children. Neuroimage 55, 165-175, doi:10.1016/j.neuroimage.2010.11.080 (2011).
14 Fair, D. A. et al. The maturing architecture of the brain's default network. Proc Natl Acad Sci U S A 105, 4028-4032 (2008).
15 Zuo, X. N. et al. Growing together and growing apart: regional and sex differences in the lifespan developmental trajectories of functional homotopy. J Neurosci 30, 15034-15043, doi:10.1523/JNEUROSCI.2612-10.2010 (2010).
16 Andrews-Hanna, J. R. et al. Disruption of large-scale brain systems in advanced aging. Neuron 56, 924-935, doi:10.1016/j.neuron.2007.10.038 (2007).
17 Greicius, M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol 21, 424-430, doi:10.1097/WCO.0b013e328306f2c5
00019052-200808000-00007 [pii] (2008).
18 Biswal, B. B. et al. Toward discovery science of human brain function. Proc Natl Acad Sci U S A 107, 4734-4739, doi:0911855107 [pii]
10.1073/pnas.0911855107 (2010).
19 Tomasi, D. & Volkow, N. D. Abnormal functional connectivity in children with attention-deficit/hyperactivity disorder. Biological psychiatry 71, 443-450, doi:10.1016/j.biopsych.2011.11.003 (2012).
20 ADHD-200-Consortium. The ADHD-200 Consortium: A Model to Advance the Translational Potential of Neuroimaging in Clinical Neuroscience. Front Syst Neurosci 6, 62, doi:10.3389/fnsys.2012.00062 (2012).
21 Mennes, M., Biswal, B. B., Castellanos, F. X. & Milham, M. P. Making data sharing work: The FCP/INDI experience. Neuroimage, doi:10.1016/j.neuroimage.2012.10.064 (in press).
